Vaccines are safe and effective. Vaccine preventable diseases are still with us, so we need to continue to protect ourselves, our children, and those around us. Clients can schedule an appointment in advance via phone for all Immunizations. No One Will Be Denied Health Services For Inability To Pay.
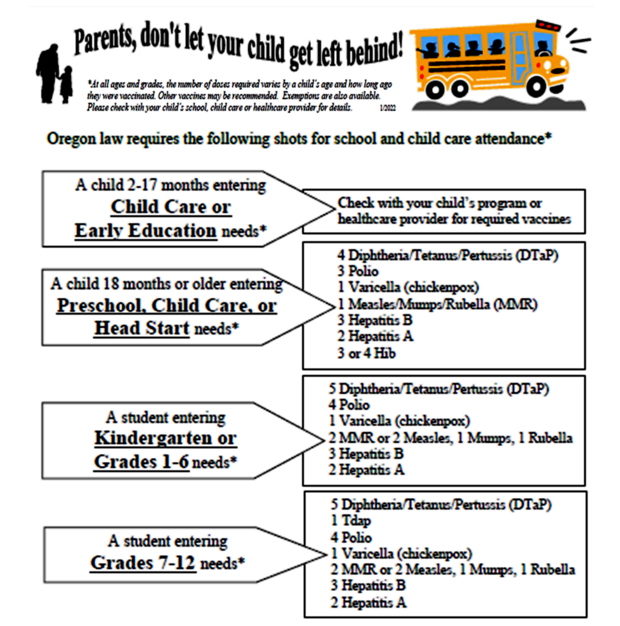
Please Note: Oregon law requires certain immunizations for school and child care attendance. At all ages and grades, the number of doses required varies by a child's age and how long ago they were vaccinated. Other vaccines may be recommended. Exemptions are also available. Please check with your child's school, child care or healthcare provider for details.
If you have additional questions or concerns, please contact us at 541-278-5432.
IMMUNIZATIONS AVAILABLE FOR:
What is diphtheria? This is an infection caused by the bacterium Corynebacterium diphtheriae and a few other Corynebacterium strains. Infection can take place in the respiratory tract and result in symptoms like fever, sore throat, difficulty swallowing, malaise, and loss of appetite. A skin infection can also occur resulting in a scaly rash or ulcers. Most diphtheria cases are caused by toxin-producing strains. The toxin is the component in the infection that causes damage to the tissue in the immediate area of infection. If left untreated, diphtheria can cause severe difficulties with breathing and swallowing (particularly in children), as well as lung or heart failure, paralysis, or death.
What vaccine can help protect me? Currently there are four vaccine types that can protect you against diphtheria: DTaP, DT, Tdap, and Td. UCo Health currently carries DTaP and Tdap. All available vaccines for diphtheria also have a formulation that helps protect against tetanus and some formulations protect against both tetanus and pertussis (whooping cough).
How does the vaccine work? Vaccination is highly protective against the toxin-producing strains of Corynebacterium. All available vaccines are considered “toxoid vaccines”, meaning they contain an inactive form of the bacterial toxin. When administered and absorbed by the body, the immune system will activate and respond to the toxoid and begin the process to make long lasting antibodies specific to the bacterial toxin. These antibodies can neutralize the toxin if you are ever exposed to the bacteria. Research shows that these vaccines are over 95% effective against diphtheria for up to 10 years. Thanks to vaccination, the United States has only 14 cases between 1996-2018 (an average of less than one case per year).
Who should get this vaccine? The CDC currently recommends the following for diphtheria vaccination:
● Infants and children through age 6: DTaP vaccination should be a total of five times with one dose at ages 2 months, 4 months, 6 months, between 15 to 18 months, and between 4 to 6 years of age. Should your healthcare clinician ad-vise avoiding the pertussis formulation, the same vaccination schedule should be followed with the DT vaccine instead.
● Individuals between ages 7 and 18: A booster shot using the Tdap vaccine is recommended at age 11 or 12. If advised to avoid the pertussis formulation, the Td vaccine should be used instead.
● Adults 19 and older: Adults should get a booster shot of the Tdap vaccine every 10 years. Adults with immune suppression are recommended to still follow the same schedule with a booster dose every 10 years. If advised to avoid the pertussis formulation, the Td vaccine should be used instead. "If you have previously been told by a healthcare provider that you should only receive the formulation without pertussis, then the same schedule would be used but with the Td vaccine instead.
Pregnant People: A booster shot of the Tdap vaccine during the third trimester of each pregnancy is recommended. This can provide protection for both the unborn baby and parent.
Vaccine Preventable Illness: Pertussis (Whooping Cough)
What is pertussis? This is a highly contagious infection caused by the bacterium Bordetella pertussis which produces several toxic or harmful products that can suppress the immune system, damage respiratory tract tissue, and induce inflammation in the lungs that reduces the ability to breathe. Pertussis is spread by inhaling the bacteria that was made airborne by an infected person coughing or sneezing. It can also be spread with prolonged time in shared breathing spaces. A common example of this is holding a newborn on your chest. People can be contagious for several weeks. Symptoms appear in 2 stages. Stage 1 includes low grade fever, nasal congestion, mild cough, and apnea (life-threatening pauses in breathing) particularly in infants and small children. Stage 2 is characterized by rapid, violent, and uncontrolled coughing fits. These coughing fits can induce vomiting during or after the fits, labored breathing, convulsions, and increased blood pressure in the brain that can cause fatal brain bleeds.
What vaccine can help protect me? Currently there are two vaccine formulations that can protect you against pertussis: DTaP and Tdap. UCo Health currently carries both DTaP and Tdap. All available vaccines for pertussis also have a formulation that also helps protect against tetanus and diphtheria.
How does the vaccine work? Vaccination is shows high efficacy for protecting against B. pertussis. All available vaccines are considered “toxoid vaccines”, meaning they contain an inactive form of the toxin or infectious component of the target bacteria. When administered and absorbed by the body, the immune system will activate and respond to the inactive bacterial components. From there it will begin the process of making long lasting antibodies specific to that bacteria. These antibodies can neutralize the pathogen and toxins if you are ever exposed to them. Research shows that DTaP prevents infection in about 98% of infants and children (which are considered high risk for disease) for the first year after vaccination and 71% 5 years after vaccination. Tdap administration to birthing people during pregnancy prevents disease for the newborn after birth in about 78% of infants younger than 2 months of age. It also prevents hospitalization in about 90% of infants under 2 months of age who have an active pertussis infection.
Who should get this vaccine? The CDC currently recommends the following for pertussis vaccination:
- Infants and children through age 6: DTaP vaccination should be a total of five times with one dose at ages 2 months, 4 months, 6 months, between 15 to 18 months, and between 4 to 6 years of age.
- Individuals between ages 7 and 18: A booster shot using the Tdap vaccine is recommended at age 11 or 12.
- Adults 19 and older: Adults should get a booster shot of the Tdap vaccine every 10 years. Adults with immune suppression are recommended to still follow the same schedule with a booster dose every 10 years.
- Pregnant people: A booster shot of the Tdap vaccine during the third trimester of each pregnancy is recommended. This can provide protection for both the unborn baby and parent.
- High-risk adults: Adults living with asthma and chronic obstructive pulmonary disease (COPD) are considered at high-risk for severe disease and should make sure their vaccination stays up to date.
**If your healthcare provider has advised you to avoid the DTaP and Tdap, be sure to ask them the best ways to protect yourself from pertussis.
Vaccine Preventable Illness: Tetanus (Lock Jaw)
What is tetanus? This is an infection caused by the bacterium Clostridioides tetani which produces a toxin that causes muscles to permanently contract, leaving them unable to relax. Symptoms of tetanus include jaw cramping/clenching, sudden, involuntary muscle spasms (often in the stomach), painfully stiff muscles all over the body, trouble swallowing, seizures, headache, fever, excessive sweating, and changes in blood pressure and heart rate. Serious health complications can arise from tetanus like broken bones, blood clots in the lungs, aspiration of saliva or vomit into the lungs resulting in an infection, breathing difficulty, and laryngospasms (uncontrolled/involuntary tightening of the vocal cords), all of which can be fatal. Tetanus does not spread from person to person. Instead, it results when bacterial spores enter through broken or damaged skin. Examples include wounds contaminated with dirt, puncture wounds like a nail or needle piercing the skin, burns, animal bites, and occasionally insect bites.
What vaccine can help protect me? Currently there are four vaccine types that can protect you against tetanus: DTaP, DT, Tdap, and Td. UCo Health currently carries DTaP and Tdap. All available vaccines for tetanus also have a formulation that helps protect against diphtheria and some formulations protect against both diphtheria and pertussis (whooping cough).
How does the vaccine work? Vaccination is extremely effective at protecting against C. tetani. All available vaccines are considered “toxoid vaccines”, meaning they contain an inactive form of the bacterial toxin. When administered and absorbed by the body, the immune system will activate and respond to the toxoid. From there it will begin the process of making long lasting antibodies specific to that toxin. These antibodies can neutralize the toxin and prevent the skeletomuscular symptoms associated with tetanus should you ever be exposed to it. In 2017, only 33 cases of tetanus were reported in the United States (more than half were reported as not being up to date on vaccination) and only resulted in 2 deaths.
Who should get this vaccine? The CDC currently recommends the following for tetanus vaccination:
● Infants and children through age 6: DTaP vaccination should be a total of five times with one dose at ages 2 months, 4 months, 6 months, between 15 to 18 months, and between 4 to 6 years of age. Should your healthcare clinician advise avoiding the pertussis formulation, the same vaccination schedule should be followed with the DT vaccine instead.
● Individuals between ages 7 and 18: A booster shot using the Tdap vaccine is recommended at age 11 or 12. If advised to avoid the pertussis formulation, the Td vaccine should be used instead.
● Adults 19 and older: Adults should get a booster shot of the Tdap vaccine every 10 years. Adults with immune suppression are recommended to still follow the same schedule with a booster dose every 10 years. If advised to avoid the pertussis formulation, the Td vaccine should be used instead.
● Pregnant people: A booster shot of the Tdap vaccine during the third trimester of each pregnancy is recommended. This can provide protection for both the unborn baby and parent.
● Injury: One of these vaccine formulations is often administered if you have a deep cut/puncture wound or have suffered an animal bite or burn.
**Note: These vaccines are typically given for injury before the 10 year mark recommended between boosters. It is safe to receive a booster dose before that 10 year mark.
Source Information:
CDC Pink Book: Epidemiology and Prevention of Vaccine Preventable Diseases
- https://www.cdc.gov/vaccines/pubs/pinkbook/dip.html
- https://www.cdc.gov/vaccines/pubs/pinkbook/pert.html
- https://www.cdc.gov/vaccines/pubs/pinkbook/tetanus.html
Textbook
- Flint, J., Racaniello, V.R., Rall, G.F., Hatziioannou, T., & Skalka, A.M. (2020). Chapter 7 - Vaccines. Principles of Virology: Volume II Pathogenesis and Control (5th edition, pp. 230-258). Washington D.C.: ASM Press. ISBN: 9781683672852
What is Hepatitis A? This is a short-term liver disease caused by the hepatitis A virus (HAV). HAV is found in the stool and blood of those who are infected. Hepatitis A is very transmittable because it requires a very small amount to establish an infection. HAV is spread from person-to-person through direct contact. Some examples of this include oral-anal sex, caring for someone who is infected, or sharing intravenous drug paraphernalia. Contamination of food with HAV can also contribute to transmission at any point in food preparation from growing, harvesting, processing, handling, and even after cooking. HAV can be spread even before symptoms appear and some infected individuals may not develop any symptoms at all. Symptoms include: Yellowing of skin or whites of the eyes, decreased appetite, nausea, vomiting, abdominal pain, fever, dark urine, light-colored stools, diarrhea, joint pain, and fatigue. Symptoms usually appear 2 to 7 weeks after infection typically last less than two months, but some people can have symptoms for as long as 6 months. In most cases of Hepatitis A, infected individuals recover with no lasting liver damage, but in some people, HAV can cause liver failure and even death.
What vaccine can help protect me? There are two hepatitis A-only vaccines approved for use in the United States: HAVRIX® and VAQTA®. There is one combination vaccine available as well called TWINRIX®, which provides protection from Hepatitis A and B. UCo Health currently carries HAVRIX® and TWINRIX®.
How does this vaccine work? The hepatitis A vaccines are categorized as inactivated vaccines. This means that they are formulated with a “killed” version of HAV. When administered, the immune system will begin the process of retaining a memory of the building blocks of HAV and producing antibodies that can neutralize those viral particles to prevent infection. Hepatitis A cases have dropped dramatically in the United States when the vaccine was introduced and case levels have continued to be low as a result of vaccination.
Who should get this vaccine? HAV vaccination is part of the routine childhood vaccines so it is important for infants and young children to be vaccinated. Vaccination is also recommended for those who are considered high risk for hepatitis A like international travelers, men who have sex with men, people who use injection drugs, people who have occupational risk for infection, people experiencing homelessness, people with HIV, and people with chronic liver disease.
● Infants and young children: Those in this category receive 2 doses. The first dose is recommended for infants who are 12 through 23 months old. The second dose should be administered at least 6 months after the first dose.
● Older children, adolescents, and adults: Those who were not vaccinated previously are still eligible for vaccination.
Source Information:
CDC Pink Book: Epidemiology and Prevention of Vaccine Preventable Diseases
Textbooks
- Flint, J., Racaniello, V.R., Rall, G.F., Hatziioannou, T., & Skalka, A.M. (2020). Chapter 7 - Vaccines. Principles of Virology: Volume II Pathogenesis and Control (5th edition, pp. 230-258). Washington D.C.: ASM Press. ISBN: 9781683672852
- Murray, P.R., Rosenthal, K.S., & Pfaller, M.A. (2016). Chapter 55 - Hepatitis Viruses. Medical Microbiology (8th edition, pp. 546-560). Philadelphia, Pennsylvania: Elsevier Inc. ISBN: 9780323299565
What is Hepatitis B? This is a liver disease caused by the hepatitis B virus (HBV). HBV is spread from person-to-person when blood, semen, vaginal secretions, amniotic fluid and some other fluids from an infected person enters the body of an uninfected or unvaccinated individual. People can become infected during birth where the mother transmits it to the baby, unprotected sex direct contact with the blood or open sores of an infected individual, or sharing needles, syringes, and drug preparation equipment. It can also be spread from shared items like toothbrushes, razors, and medical equipment like glucose monitors. Infection can be acute (short-term) or chronic (permanent). Symptoms of acute infection include: Yellowing of skin or whites of the eyes, decreased appetite, nausea, vomiting, abdominal pain, fever, dark urine, clay-colored stools, joint pain, and fatigue. Symptoms, if they do appear, usually usually show up about 3 months after infection begins and typically last for several weeks, but some people can have symptoms for as long as 6 months. In chronic hepatitis B, there are usually no symptoms for decades but if they do appear they are similar to acute infection symptoms. About 1 in 4 people with hepatitis B become chronically infected during childhood and about 15% of those who become chronically infected after childhood will eventually die from liver conditions. Chronic HBV infection often results in cirrhosis (scarring of the liver) or liver cancer
What vaccine can help protect me? There are three hepatitis B-only vaccines approved for use in the United States: Engerix-B®, Heplisav-B®, and Recombivax HB®. There are three combination vaccines available as well called TWINRIX®, which provides protection from Hepatitis A and B, Pediarix® which provides protection from diphtheria, tetanus, pertussis, polio, and hepatitis B, and Vaxelis® which protects against the same diseases as Pediarix® in addition to Haemophilus influenzae B. UCo Health currently carries Engerix-B® and all three combination vaccines.
How does this vaccine work? The hepatitis B vaccines are categorized as subunit vaccines. That means they are formulated with a building block piece of the virus called the Surface Antigen protein. When administered, the immune system will begin the process of retaining a memory of the HBV Surface Antigen protein and producing antibodies that can neutralize those viral particles to prevent infection. Since HBV vaccination was first introduced in 1982, reports of acute HBV infections have declined by approximately 90%.
Who should get this vaccine? HBV vaccination is part of the routine childhood vaccines so it is important for infants and young children to be vaccinated. Vaccination is also recommended for those who are considered high risk for hepatitis A like international travelers, those who have unprotected sex, people who use injection drugs, people who have occupational risk for infection, people experiencing homelessness, people with HIV, and people with chronic health issues like liver disease, kidney disease, and elderly individuals with diabetes.
- Infants and young children:Those in this category receive 3 doses at variable dosing schedules depending on age and vaccine brand.
- Older children, adolescents, and adults: Those who were not vaccinated previously are still eligible for vaccination and will have a different vaccination schedule depending on age and vaccine brand.
Source Information:
CDC Pink Book: Epidemiology and Prevention of Vaccine Preventable Diseases
Textbooks
- Flint, J., Racaniello, V.R., Rall, G.F., Hatziioannou, T., & Skalka, A.M. (2020). Chapter 7 - Vaccines. Principles of Virology: Volume II Pathogenesis and Control (5th edition, pp. 230-258). Washington D.C.: ASM Press. ISBN: 9781683672852
- Murray, P.R., Rosenthal, K.S., & Pfaller, M.A. (2016). Chapter 55 - Hepatitis Viruses. Medical Microbiology (8th edition, pp. 546-560). Philadelphia, Pennsylvania: Elsevier Inc. ISBN: 9780323299565
What is Measles? Measles is a very contagious disease caused by the measles virus. Measles is a systemic infection, meaning it affects many areas all over the body. Transmission occurs person-to-person through large respiratory droplets and airborne aerosolized droplets. If one person has measles, up to 90% of people close to them will also become infected if not immune from prior infection or vaccination. The incubation period from exposure to symptom onset takes about 11-12 days. The symptoms that typically appear first include a fever that can climb very high (peaking as high as 103°F-105°F), cough, nasal congestion, Koplik spots (white but often reddened spots inside the mouth), and red watery eyes like pinkeye. A rash will begin to form typically starting on the face and then spreading to the rest of the body about 14 days after exposure. Rash is characterized by flat red spots on white skin but may be harder to see on darker skin tones, typically is not itchy, and lasts about 5-6 days. Common complications from infections include ear infections and diarrhea. Severe complications include pneumonia (1 in 20 child cases) and swelling of the brain (1 in 1,000 child cases) which can lead to seizures, deafness, and/or intellectual disabilities. About 1 in 5 unvaccinated measles cases are hospitalized. Complications during pregnancy can occur and result in premature birth and low-birth weight. Before the introduction of MMR vaccine in 1963, the United States had an estimated 3-4 million measles cases each year, where 400-500 of these cases died, 48,000 were hospitalized, and 1,000 developed encephalitis. Between 2020-2022, there were a total of 180 measles cases reported showing the effectiveness of vaccination.
What is Mumps? Mumps is an infection of the salivary glands caused by the mumps virus. Transmission occurs person-to-person through respiratory droplets and saliva. The incubation period from exposure to symptom onset is usually around 16-18 days. The first symptoms to appear are typically nonspecific but include body aches, decreased appetite, fatigue, headache, and low-grade fever. Soon after salivary gland swelling occurs and lasts for about 5 days, often resulting in an earache and tenderness of the jaw. Complications of mumps can involve many different areas of the body. Some complications include inflammation of the testicles, ovaries, breast tissue, pancreas, tissue surrounding the brain and spinal cord (meningitis), and swelling of the actual brain itself. Meningitis can result in deafness, seizures, and permanent drooping of facial muscles. Inflammation of the testicles could lead to temporary sterility. Mumps complications are most common in adults rather than children. Before the first mumps vaccine was licensed in 1967, the United States had about 152,000 cases each year. By 1985, less than 3,000 cases were reported annually.
What is Rubella? Rubella is a contagious disease similar to measles caused by the Rubella virus. Transmission occurs person-to-person through respiratory droplets. The incubation period from exposure to symptom onset is usually around 14 days. The first symptoms to appear are typically a low-grade fever, headache, red watery eyes, swollen lymph nodes, cough, nasal congestion, and a rash that looks red or pink spots on white skin, but may be harder to see on darker skin tones. The rash may be rough or bumpy and may be slightly itchy. The rash usually starts on the head and neck, and then may spread to other parts of the body. Complications of rubella are rare, but about 1 in 3,000 have blood disorder issues that develop like thrombocytic purpura which is autoimmune based and can prevent the blood from forming clots resulting in easy bruising or bleeding. Swelling in the brain occurs in about 1 in 6,000 cases and can be fatal. If rubella infection occurs during pregnancy, virus can be passed to the fetus and result in miscarriages, stillbirths, and severe birth defects. Prior to vaccine introduction, in 1964-1965, the United States had an estimated 12.5 million rubella cases, 11,000 pregnant people lost their babies, 2,100 newborns died, and 20,000 babies were born with severe birth defects. Today, there are fewer than 10 cases reported each year in the U.S.
What vaccine can help protect me? The MMR (measles, mumps, and rubella) vaccine protects against all three diseases. There are currently two MMR vaccine brands available: M-M-R II® and PRIORIX®. There is also a MMRV vaccine available called ProQuad® that protects against measles, mumps, rubella, and varicella (chickenpox). UCoHealth currently carries M-M-R II® and ProQuad®. One dose of MMR vaccine is 93% effective against measles, 78% effective against mumps, and 97% effective against rubella. Two doses are 97% effective against measles and 88% effective against mumps.
How do the vaccines work? The MMR vaccine is a live attenuated virus vaccine meaning it contains live versions of all three viruses, however the likelihood of an infection coming from vaccination is extremely low because the virus is weakened so our immune systems can quickly deal with it before it has a chance to cause infection. Also, the amount of virus contained in the vaccine is small, so first responder immune cells find and kill them before they have a chance to cause illness. The vaccine allows the immune system to easily identify the viruses and create antibodies that neutralize them.
Who should get vaccinated? The MMR vaccine is part of routine childhood immunization.
● Children 12 months to 6 years old: Vaccination is given in 2 doses: First dose at age 12-15 months, second dose at age 4-6 years old. Although, children can receive the second dose earlier as long as it is at least 28 days after the first.
● Post high school educational students: If not vaccinated earlier or do not have presumptive evidence of immunity, two doses of MMR vaccine are required, separated by at least 28 days.
● Adults: Adults who were vaccinated earlier or do not have presumptive evidence of immunity should get at least one dose of MMR vaccine. Some adults may need two doses if they are a healthcare worker or international traveler.
Source Information:
CDC Pink Book: Epidemiology and Prevention of Vaccine Preventable Diseases
- https://www.cdc.gov/vaccines/pubs/pinkbook/meas.html
- https://www.cdc.gov/vaccines/pubs/pinkbook/mumps.html
- https://www.cdc.gov/vaccines/pubs/pinkbook/rubella.html
Textbooks
- Flint, J., Racaniello, V.R., Rall, G.F., Hatziioannou, T., & Skalka, A.M. (2020). Chapter 7 - Vaccines. Principles of Virology: Volume II Pathogenesis and Control (5th edition, pp. 230-258). Washington D.C.: ASM Press. ISBN: 9781683672852
- Murray, P.R., Rosenthal, K.S., & Pfaller, M.A. (2016). Chapter 52- Tongaviruses and Flaviviruses. Medical Microbiology (8th edition, pp. 511-521). Philadelphia, Pennsylvania: Elsevier Inc. ISBN: 9780323299565
What is Poliomyelitis? Poliomyelitis, commonly referred to just as “polio”, is a disabling and potentially deadly disease caused by the poliovirus. This virus is very contagious and enters the body through the mouth. It is spread through contact with feces of an infected person either by means of feces contamination on your hands and then touching your mouth or by putting objects in your mouth that are contaminated. Transmission can also from respiratory droplets, like from a sneeze or cough, from an infected person, however this route is much less common. Most people who get infected will not have any visible symptoms. About 1 in 4 cases will have flu-like symptoms which typically last around 2 to 5 days, and then go away on their own. A smaller portion of cases will develop more serious symptoms that can affect the brain and spinal cord. Meningitis, which is inflammation of the tissue that lines the brain and spinal cord, can occur in 1 to 5% of polio cases, depending on the virus type. Paralysis or severe weakening of the extremities occurs in about 1 out of 200 people depending on the virus type. Between 2 to 10% of polio cases with paralysis leads to death because the muscles that help with breathing fail to work. Children who have recovered from polio may experience post-polio syndrome, which is characterized by new muscle pain, weakness, or paralysis as adults nearly 15 to 40 years later. There is no cure of paralytic polio and no specific treatment.
What vaccine can help protect me? The enhanced potency inactivated polio vaccine (eIPV) called IPOL® has been the only polio vaccine used in the United States since 2000. Combination vaccines are also used like Pentacel® (DTaP-IPV/Hib), Pediarix® (DTaP-IPV-HepB), Kinrix® (DTaP-IPV), VAXELIS® (DTaP-IPV-Hib-HepB), and Quadracel® (DTaP-IPV), all of which protect against polio in addition to other diseases. While vaccines help us build a strong immune system, good hygiene is one of the best ways to avoid the disease. Washing hands often with warm soapy water, especially after engaging with high touch surfaces is essential to help prevent the disease. Alcohol-based hand sanitizers do not kill the poliovirus, so actual handwashing with soap is the best.
How do the vaccines work? The IPV is considered a whole-inactivated viral vaccine, meaning it contains whole-virus that has been inactivated or killed so that it cannot cause infection, but can be identified by the immune system. From there, the immune system can create antibodies that function to neutralize the virus if it were to ever enter the body.
Who should get vaccinated? Preteens and teens are recommended to get this as they are considered high risk.
● Infants and young children: IPV is part of the routine childhood immunizations recommended to get four doses, one at each of the following ages: 2 months, 4 months, 6-18 months, and 4-6 years of age.
● Adults: Most adults have already been vaccinated against polio during childhood, so if you were raised in the United States, it is safe to assume that you were vaccinated. For those adults who may have been raised in a different country and know that you were not vaccinated, or are vaccinated but traveling to a country with increased risk of exposure to polio should talk with their healthcare provider about vaccination.
Source Information:
CDC Pink Book: Epidemiology and Prevention of Vaccine Preventable Diseases
Textbooks
- Flint, J., Racaniello, V.R., Rall, G.F., Hatziioannou, T., & Skalka, A.M. (2020). Chapter 7 - Vaccines. Principles of Virology: Volume II Pathogenesis and Control (5th edition, pp. 230-258). Washington D.C.: ASM Press. ISBN: 9781683672852
- Murray, P.R., Rosenthal, K.S., & Pfaller, M.A. (2016). Chapter 55 - Hepatitis Viruses. Medical Microbiology (8th edition, pp. 546-560). Philadelphia, Pennsylvania: Elsevier Inc. ISBN: 9780323299565
What is Varicella? Varicella, more commonly known as chickenpox, is a highly contagious disease caused by the varicella-zoster virus (VZV). Symptoms of infection include an itchy, blister-like rash that typically first show up on the chest, back, and face, but then spreads over the entire body, sometimes including the inside of the mouth, eyelids, and/or genital area. The blisters are fluid-filled initially, but turn to scabs after about 1 week. Other symptoms include fever, fatigue, loss of appetite, and headache. Illness usually lasts about 4 to 7 days. While typically not life-threatening for most, varicella can be severe or fatal in babies and those with weakened immune systems. Serious complications from varicella include bacterial infections of the skin, pneumonia, infection or swelling of the brain, bleeding problems, bloodstream infections, and severe dehydration. There are also large risks during pregnancy. VZV is typically spread through direct contact with the lesions of an infected individual. It is estimated that if one person has varicella, up to 90% of the people close to that person who are not vaccinated or immune will become infected. It takes about 2 weeks after exposure for symptoms to develop. VZV can also cause shingles later on in life because the virus remains dormant in the body after a chickenpox infection. Reactivation of the virus causes pain, itching, or tingling of the skin followed by a very painful rash of small blister-like sores, typically on one side of the body often on the torso or face.
What vaccine can help protect me? One varicella-only vaccine called Varivax® is available in the United States. One combination vaccine called ProQuad® (MMRV) is also available in the U.S. and protects against varicella as well as measles, mumps and rubella. UCo Health carries both Varivax® and ProQuad®.
How do the vaccines work? Varicella vaccines are categorized as live-attenuated virus vaccines. This means that they do contain live VZV, however, the virus has been significantly weakened. The likelihood of an infection coming from vaccination is extremely low because the virus is weakened so our immune systems can quickly deal with it before it has a chance to cause infection, as well as the fact that the amount of virus contained in the vaccine is small so first responder immune cells find and kill them before they have a chance to cause illness. The vaccine allows the immune system to easily identify the virus and create antibodies that neutralize it.
Who should get vaccinated? Varicella vaccination is a part of the routine childhood immunizations.
● Children 12 months to 12 years old: Vaccination is given in 2 doses: First dose at age 12-15 months, second dose at age 4-6 years old. For the first dose, CDC recommends that the MMRV be given separately in children 12-47 months old. Second doses between 15 months-12 years old and first dose for those older than 48 months, the MMRV vaccine is generally preferred.
● People 13 years or older: If not vaccinated earlier, the varicella-only vaccine is recommended in 2 doses 4-8 weeks apart. MMRV vaccine is not approved for people in this age group.
Source Information:
CDC Pink Book: Epidemiology and Prevention of Vaccine Preventable Diseases
Textbooks
- Flint, J., Racaniello, V.R., Rall, G.F., Hatziioannou, T., & Skalka, A.M. (2020). Chapter 7 - Vaccines. Principles of Virology: Volume II Pathogenesis and Control (5th edition, pp. 230-258). Washington D.C.: ASM Press. ISBN: 9781683672852
- Murray, P.R., Rosenthal, K.S., & Pfaller, M.A. (2016). Chapter 43 - Human Herpesviruses. Medical Microbiology (8th edition, pp. 425-446). Philadelphia, Pennsylvania: Elsevier Inc. ISBN: 9780323299565
What is Hib disease? This is the name for any illness caused by the bacterium Haemophilus influenzae type b (Hib). Hib is spread from person to person primarily through respiratory droplets produced when an infected person or Hib carrier coughs, sneezes, or talks and those droplets come in contact with another person's eyes, mouth, or nose. Hib can also be spread through contact with contaminated surfaces or objects, although less common. Typically, H. influenzae lives in people’s noses and throats, usually causing no harm. However, if present in someone with a weakened or under-developed immune system, it can easily spread to other areas of the body and cause severe illness. Contrary to its name, Hib does not cause the flu. The most severe forms of infection can present as pneumonia, bloodstream infection, or meningitis. Symptoms can include fever and chills, shortness of breath, chest pain, headache, body aches, fatigue, nausea, diarrhea, pain in the belly, altered mental status, stiff neck, and light sensitivity. In infants and young children, Hib can cause epiglottitis which is a life-threatening condition where the tissue that covers the airway when swallowing becomes inflamed and essentially cuts off the ability to breathe.
What vaccine can help protect me? There are three “Hib-only” vaccines available: PedvaxHIB®, ActHIB®, and Hiberix®. Additionally, there are two combination vaccines available: Pentacel® and Vaxelis™. UCo Health currently carries PedvaxHIB® Hib-only vaccine, and both Pentacel® and Vaxelis™ combination vaccines.
How do the vaccines work? The Hib vaccines are considered polysaccharide conjugate vaccines. They contain a set of biomolecules called polysaccharides, which are the building blocks of the protective capsule employed by H. influenzae. These polysaccharide vaccine components are then conjugated onto a carrier protein to aid in delivery and boost the immune response to the polysaccharide. This will stimulate the production of antibodies toward the capsule on the exterior of the H. influenzae bacterium. The combination vaccines follow this same principle but also vaccinate against other diseases at the same time. Pentacel® contains a freeze dried form of ActHIB® that gets redissolved in a liquid solution of DTaP/IPV (Diphtheria, Tetanus, and Pertussis vaccine, as well as inactivated polio vaccine). Vaxelis™ contains Pentacel® that uses PedvaxHIB® instead of ActHIB® for the Hib component, and also contains Recombivax HB® which is a vaccine used for Hepatitis B.
Who should get this vaccine? All infants, including preterm infants, should receive a primary series beginning at 2 months of age. The number of doses depends on the brand of vaccine used:
● If ActHIB®, Hiberix®, Pentacel®, or Vaxelis™ is used, 3 doses should be administered - 1 dose at 2 months, 1 dose at 4 months, and 1 dose at 6 months of age.
● If PedvaxHIB® is used, 2 doses should be administered - 1 dose at 2 months and 1 dose at 4 months of age.
● A booster dose is recommended at 12 to 15 months of age for all infants. ActHIB®, Hiberix®, Pentacel®, or PedvaxHIB® can be used for the booster dose, but Vaxelis™ should not be used for a booster.
Talk to your healthcare provider about which vaccine is right for you or your child.
Source Information:
CDC Pink Book: Epidemiology and Prevention of Vaccine Preventable Diseases
Textbooks
- Flint, J., Racaniello, V.R., Rall, G.F., Hatziioannou, T., & Skalka, A.M. (2020). Chapter 7 - Vaccines. Principles of Virology: Volume II Pathogenesis and Control (5th edition, pp. 230-258). Washington D.C.: ASM Press. ISBN: 9781683672852
- Murray, P.R., Rosenthal, K.S., & Pfaller, M.A. (2016). Chapter 11 - Antimicrobial Vaccines. Medical Microbiology (8th edition, pp. 97-103). Philadelphia, Pennsylvania: Elsevier Inc. ISBN: 9780323299565
What is COVID-19? This is an infection caused by the highly contagious SARS-CoV-2 virus, of which several variants currently exist. Because of the existence of multiple variants of the virus, a person can get COVID multiple times. COVID is spread from person to person primarily through respiratory droplets produced when an infected person coughs, sneezes, or talks and it comes in contact with another person's eyes, mouth, or nose. COVID can also be spread through contact with contaminated surfaces or objects. Symptoms can range from mild to severe and some may not have any symptoms but can still spread the virus without knowing they have it. Symptoms include fever or chills, cough, shortness of breath, fatigue, muscle or body aches, headache, new loss of taste or smell, sore throat, nasal congestion or runny nose, nausea, vomiting, and/or diarrhea.
What vaccine can help protect me? Currently available are the mRNA vaccines from either Pfizer-BioNTech or Moderna, as well as a protein subunit vaccine from Novavax. Booster doses of COVID-19 vaccines will be needed with the emergence of new variants of the virus that make the vaccines less effective over time.
How do the vaccines work? (1) The mRNA vaccines for COVID involve a specialized system to deliver a small piece of mRNA that teaches your cells to make the same protein that is found on the outside of SARS-CoV-2, called the Spike Protein. Production of this protein helps train the immune system to identify it and produce neutralizing antibodies that prevent it from working correctly. This protein helps the virus enter our cells and perpetuate infection so presence of neutralizing antibodies blocks the protein’s ability to function and inhibits the virus from entering cells and causing infection. While mRNA is considered genetic material, the mRNA molecules in the vaccine do not and cannot affect or interact with human DNA. (2) The protein subunit vaccine for COVID contains small fragments of the viral Spike Protein. Just like with mRNA vaccines, the immune system will identify these pieces of protein to create antibodies that target those specific components and inhibit the infectious potential of the virus. In protein subunit vaccines, the formula also contains an adjuvant, which is a substance that helps boost the immune system response to the vaccine components since protein subunit vaccines do not have the greatest immunogenicity. Matrix-M is the adjuvant used in Novavax vaccine and is derived from Quillaja saponins, which are naturally occurring compounds in the bark of Soapbark trees commonly found in Chile. (3) The viral vector vaccine for COVID uses a modified, nonreplicating version of a different harmless virus to deliver instructions to our cells to produce the Spike Protein of SARS-CoV-2 and yet again stimulate the production of antibodies towards that protein like the previously mentioned vaccines.
Who should get this vaccine? The CDC currently recommends the following for COVID-19 vaccination:
● Anyone 6 months of age or older is eligible for vaccination against COVID-19 with the Moderna or Pfizer-BioNTech vaccines.
● Anyone 12 years or older is eligible for vaccination against COVID-19 with the Novavax vaccine.
● Anyone 18 years or older is eligible for vaccination against COVID-19 with the J&J/Janssen vaccine.
Talk to your healthcare provider about which vaccine is right for you. For any questions about when you should get a booster dose, you can call UCo Health or talk with your provider for booster recommendations.
Source Information:
CDC Overview of COVID-19 Vaccines
Textbook
- Murray, P.R., Rosenthal, K.S., & Pfaller, M.A. (2016). Chapter 47 - Coronaviruses and Noroviruses. Medical Microbiology (8th edition, pp. 469-474). Philadelphia, Pennsylvania: Elsevier Inc. ISBN: 9780323299565
What is HPV? HPV is the abbreviation for the human papillomavirus. Infection with this virus is common and nearly everyone will get HPV at some point during their life. There are many forms of the virus that are not associated with disease, but there are HPV types that can cause disease. This virus is associated with development of common warts, plantar warts, flat warts, and genital warts. HPV is spread through skin-to-skin contact or skin contact with contaminated areas like showers, pool decks, or locker rooms for common warts and plantar warts. Genital warts are sexually transmitted through vaginal, anal, or oral sex. You can still contract the virus from someone even if they do not have any signs or symptoms. Most HPV infections go away by themselves within 2 years, but some infections can last longer. Other types of HPV are associated with the development of several different types of cancers like cervical, vaginal, vulvar, penile, anal, and oropharyngeal cancers. In the United States, HPV causes about 36,000 cases of cancer each year.
What vaccine can help protect me? Since 2017, only Gardasil®-9 is the approved HPV vaccine in the United States. This vaccine covers nine different HPV types: 6, 11, 16, 18, 31, 33, 45, 52, and 58. These are the strains that are associated with the majority of HPV-related cancers. Cervical cancer was once the leading cause of cancer deaths among women in the United States, but since the introduction of HPV vaccines and cervical cancer screenings, it has now become one of the most preventable cancers.
How does this vaccine work? Gardasil®-9 is a non-infectious recombinant vaccine that contains virus-like particles from the major capsid protein L1 (a building block of HPV that helps it enter human cells to infect them). The immune system will begin the process of retaining a memory of these virus-like particles and produce antibodies towards the HPV L1 protein to neutralize it, preventing HPV infection from starting or if you already have HPV, can help neutralize HPV that is trying to spread to new cells. Early protection works best! That is why the HPV vaccine is recommended earlier rather than later because it provides protection before ever having contact with the virus.
Who should get this vaccine?
- Those between 9 and 14 years old:HPV vaccination is routinely recommended at age 11 or 12 years although can be started at age 9. If started before the 15th birthday, the 2-dose regimen is recommended with one dose to start followed by another dose between 6 and 12 months later.
- Those between 15 and 26 years old: HPV vaccination is recommended for anyone through age 26 who were not adequately vaccinated earlier. The 3-dose regimen is recommended with one dose to start, followed by a second dose 2 months later, and then a third dose 6 months from the original dose.
- Adults ages 27 through 45 years: Although the HPV vaccine is FDA approved to be given through age 45 years, HPV vaccination is not recommended for all adults ages 27 through 45 years. Instead, those in this age bracket should talk with your healthcare provider about HPV vaccination because most in this group receive less benefit because most have already been exposed to the virus.
**Note: HPV vaccination is not recommended during pregnancy because it has not been studied in pregnant people in its clinical trials. Discuss with your healthcare provider about vaccination and pregnancy.
Source Information:
CDC Pink Book: Epidemiology and Prevention of Vaccine Preventable Diseases
Textbooks
- Flint, J., Racaniello, V.R., Rall, G.F., Hatziioannou, T., & Skalka, A.M. (2020). Chapter 7 - Vaccines. Principles of Virology: Volume II Pathogenesis and Control (5th edition, pp. 230-258). Washington D.C.: ASM Press. ISBN: 9781683672852
- Murray, P.R., Rosenthal, K.S., & Pfaller, M.A. (2016). Chapter 41 - Papillomaviruses and Polyomaviruses. Medical Microbiology (8th edition, pp. 408-417). Philadelphia, Pennsylvania: Elsevier Inc. ISBN: 9780323299565
What is Influenza? Influenza is a respiratory illness, often referred to as “the flu”, caused by the influenza virus. There are several types of influenza but strains A and B are the most common that cause disease in humans. Influenza B is categorized into two lineages: B/Yamagata and B/Victoria There are two proteins within the virus that help categorize the subtypes of influenza A and B strains. Hemagglutinin (HA or H) and neuraminidase (NA or N). There are 8 different H subtypes (H1, H2, H3, H5, H6, H7, H9, & H10) and 6 different N subtypes (N1, N2, N6, N7, N8, & N9) that have been identified in humans. Different combinations of these subtypes help distinguish which influenza virus is causing infection (ex: H1N1 or H3N2). Changes to the HA and NA proteins cause the virus to appear slightly different to the immune system, causing immunity to drop, which is why yearly vaccination is suggested to account for the most common circulating strains. Abrupt changes in H or H-N combinations result in the emergence of influenza pandemics like was seen in 2009-2010 with the “swine flu”. Transmission occurs person-to-person through respiratory droplets or small aerosolized droplets. It can also occur through direct or indirect contact with respiratory secretions on contaminated surfaces or objects. Symptoms of influenza are sudden and include cough, sore throat, and nasal congestion, fever/chills, headache, and body aches. Children may also experience vomiting and diarrhea. Symptoms usually last for about a week. Most influenza cases resolve with no issues, but serious infection can occur leading to complications like secondary bacterial pneumonia, ear infections, bronchitis, or exacerbation of underlying respiratory medical conditions. Most deaths due to influenza occur in people 65 years or older.
What vaccine can help protect me? Three types of influenza vaccines are available in the United States: inactivated influenza vaccine (IIV); live, attenuated influenza vaccine (LAIV); and recombinant influenza vaccine (RIV). Trivalent vaccine contains three inactivated viruses: type A(H1N1), type A(H3N2), and type B (either B/Yamigata or B/Victoria). Quadrivalent influenza vaccines were first introduced during the 2013–2014 season. They contain the same antigens as trivalent vaccines, with an additional type B strain. Most vaccines administered in the United States are quadrivalent. UCo Health carries all three types of influenza vaccines.
How do the vaccines work? IIVs typically contain inactivated split virus meaning they contain inactivated or dead virus for four influenza strains (2 A and 2 B types). IIVs are administered as an intramuscular injection. RIVs contain synthetically made HA proteins that can then be introduced into the body through intramuscular injection. Lastly, LAIVs contain live influenza virus that has been drastically weakened and is introduced into the body through a nasal spray in each nostril. The likelihood of an infection coming from vaccination is extremely low because the virus is weakened so our immune systems can quickly deal with it before it has a chance to cause infection, as well as the fact that the amount of virus contained in the vaccine is small so first responder immune cells find and kill them before they have a chance to cause illness. All of these vaccines teach the immune system to recognize the HA protein and produce antibodies that will neutralize that target and disable the virus.
Who should get vaccinated? Anyone 6 months or older is eligible for influenza vaccination. Those with underlying chronic health conditions or those 65 and older are strongly recommended to get vaccinated to decrease the likelihood of severe infection should it be contracted. Vaccination is recommended each fall, but it is never too late to get vaccinated!
- IIV or RIV: IIVs and RIVs for the 2022-2023 season can be administered to anyone 6 months of age or older. Specific brands are specified for those in certain age ranges or who have compromised immune systems or chronic underlying health conditions. Your healthcare provider will select the brand that fits you the best based on these criteria. If you have an allergy to eggs should mention this prior to vaccination, as some influenza vaccines do contain egg proteins in their formulations.
- LAIV: All nasal spray influenza vaccines for the 2022-2023 season are quadrivalent and can be administered to anyone 2 to 49 years old with no chronic health conditions. This vaccine should be avoided for those under 2 years old, 50 years or older, have suppressed immune systems, or are pregnant.
**For questions about what vaccine is right for you, talk to your healthcare provider or call UCo Health for booster recommendations.
Source Information:
CDC Pink Book: Epidemiology and Prevention of Vaccine Preventable Diseases
Textbooks
- Flint, J., Racaniello, V.R., Rall, G.F., Hatziioannou, T., & Skalka, A.M. (2020). Chapter 7 - Vaccines. Principles of Virology: Volume II Pathogenesis and Control (5th edition, pp. 230-258). Washington D.C.: ASM Press. ISBN: 9781683672852
- Murray, P.R., Rosenthal, K.S., & Pfaller, M.A. (2016). Chapter 49 - Orthomyxoviruses. Medical Microbiology (8th edition, pp. 487-495). Philadelphia, Pennsylvania: Elsevier Inc. ISBN: 9780323299565
What is Meningococcal Disease? This is a disease caused by the bacterium known as Neisseria meningitidis. This type of bacteria can be spread from person-to-person when people share respiratory secretions like saliva through coughing or kissing. It can also be spread through close or lengthy contact so those who share the same household as someone with meningococcal meningitis are at greater risk. This disease cannot be spread through casual contact. Infection can present in two forms: meningitis and septicemia. Meningitis is defined as inflammation of the tissue that lines the brain and spinal cord. Septicemia is defined as an infection that is within the bloodstream. In meningococcal disease, these forms can occur on their own, or more commonly, both together. The most common symptoms of meningococcal meningitis infection include fever, headache, and stiff neck. Some additional symptoms may include nausea, vomiting, eyes being more sensitive to light, and/or altered mental status. Symptoms of meningococcal septicemia include fever/chills, fatigue, vomiting, cold hands and feet, body aches, diarrhea, and in the later stages, a dark purple rash. Meningococcal infections are very serious and can become deadly within a matter of hours. It is treatable with antibiotics, however, between 10-15% of cases will die even with antibiotic treatment. Up to 1 in 5 survivors will have long-term disabilities like loss of limb(s), deafness, brain damage, and other nervous system problems.
What vaccine can help protect me? There are two types of meningococcal vaccines licensed in the United States: Meningococcal conjugate (MenACWY) and Serogroup B meningococcal (MenB) vaccines. UCo Health currently carries MenQuadFi® which is a meningococcal conjugate vaccine. Since 2005, when the MenACWY vaccine was introduced in the United States, rates of meningococcal disease in teens caused by serogroups C, Y, and W have decreased by over 90% (note: serogroup A meningococcal disease continues to be very rare in the United States).
How do the vaccines work? The MenACWY vaccines contain a set of biomolecules called polysaccharides, which are the building blocks of the protective layer on the outside of the N. meningitidis bacterium. These polysaccharide vaccine components are then conjugated onto a carrier protein to aid in delivery and boost the immune response to the polysaccharide. MenACWY vaccines protect against four serogroups (categories) of N. meningitidis as the name suggests; types A, C, W, and Y. This will stimulate the production of antibodies towards the bacterium. The MenB vaccines specifically target the B type bacterium and are categorized as recombinant protein vaccines. This means it contains several important proteins that either make up the N. meningitidis bacterium or are used by the bacterium to promote disease. Four proteins are used in the formulation and help the body to recognize these components to create antibodies that will disarm and kill the bacterium.
Who should get vaccinated? Preteens and teens are recommended to get this as they are considered high risk.
● Preteens and Teens: All 11 to 12 year olds should get a MenACWY, with a booster dose at 16 years old. Some teens may also get a MenB vaccine, preferably at age 16 to 18 years old. While some may choose to get a MenB vaccine, certain preteens and teens should get it if they have a rare type of immune disorder called complement component deficiency, are taking complement inhibitor medications, have HIV, have a damaged spleen, sickle cell disease, or have had their spleen removed, or are part of a population deemed at high risk due to a serogroup B meningococcal disease outbreak.
● Children 2 months to 10 years of age: MenACWY vaccination is only recommended if the child has complement component deficiency, is taking complement inhibitor medications, has a damaged spleen, sickle cell disease, or has had their spleen removed, have HIV, or are part of a population deemed at high risk due to a meningococcal disease A, C, W, or Y outbreak or traveling to an area where meningococcal disease is common. MenB vaccination is only recommended for the reasons listed above or the child is at high risk due to serogroup B meningococcal disease outbreak.
● Adults: MenACWY and/or MenB vaccination is only recommended for the reasons previously stated above. Vaccination may be recommended for those starting college who are not up-to-date on the vaccine or for job related exposures like researchers who are routinely exposed to N. meningitidis or are a military recruit.
Source Information:
CDC Pink Book: Epidemiology and Prevention of Vaccine Preventable Diseases
Textbooks
- Flint, J., Racaniello, V.R., Rall, G.F., Hatziioannou, T., & Skalka, A.M. (2020). Chapter 7 - Vaccines. Principles of Virology: Volume II Pathogenesis and Control (5th edition, pp. 230-258). Washington D.C.: ASM Press. ISBN: 9781683672852
- Murray, P.R., Rosenthal, K.S., & Pfaller, M.A. (2016). Chapter 23 - Neisseria and Related Genera. Medical Microbiology (8th edition, pp. 234-242). Philadelphia, Pennsylvania: Elsevier Inc. ISBN: 9780323299565
What is Rotavirus Gastroenteritis? Rotavirus gastroenteritis is an infection caused by the rotavirus in the stomach and intestines. Transmission occurs person-to-person through the fecal-oral route or can be acquired through ingestion of contaminated food and water, or contact with contaminated surfaces or objects. The incubation period from exposure to symptom onset is usually less than 48 hours. The symptoms can vary in severity depending whether it is the first infection or reinfection. The infection can be asymptomatic, may cause mild watery diarrhea, or can result in severe dehydrating diarrhea with fever and vomiting. First infection after 3 months of age is generally the most severe, but gastrointestinal symptoms typically resolve in 3-7 days. In infants and young children severe diarrhea can lead to dehydration, electrolyte imbalance, and metabolic acidosis from kidney damage or failure. Infants can dehydrate quickly and if not adequately treated can be fatal.
What vaccine can help protect me? Two rotavirus vaccines are licensed in the United States: RotaTeq® and Rotarix®. UCo Health currently carries Rotarix®. Prior to vaccine introduction, an estimated 2.7 million cases occurred per year in the United States, with about 95% of children infected by age 5 years old. After vaccine introduction an average of 280,000 clinic visits, 62,000 emergency department visits, and 45,000 hospitalization have been averted.
How do the vaccines work? Both available vaccines are categorized as live attenuated virus vaccines meaning they contain live versions of the rotavirus, however the likelihood of an infection coming from vaccination is extremely low because the virus is weakened, so our immune systems can quickly deal with it before it has a chance to cause infection. Also, the amount of virus contained in the vaccine is small, so first responder immune cells find and kill the virus before it has a chance to cause illness. The vaccine allows the immune system to easily identify the virus and create antibodies that neutralize it. These vaccines are not given through the typical “shot” method. Instead, they are given orally in a small, swallowable amount for an infant.
Who should get vaccinated? The rotavirus vaccine is part of the routine childhood immunizations.
● Infants: Vaccination with RotaTeq® is given in 3 doses: First dose at 2 months, second dose at 4 months, and third dose at 6 months of age. Vaccination with Rotarix® is given in 2 doses: First dose at 2 months, second dose at 4 months of age.
Source Information:
CDC Pink Book: Epidemiology and Prevention of Vaccine Preventable Diseases
Textbooks
- Flint, J., Racaniello, V.R., Rall, G.F., Hatziioannou, T., & Skalka, A.M. (2020). Chapter 7 - Vaccines. Principles of Virology: Volume II Pathogenesis and Control (5th edition, pp. 230-258). Washington D.C.: ASM Press. ISBN: 9781683672852
- Murray, P.R., Rosenthal, K.S., & Pfaller, M.A. (2016). Chapter 51 - Reoviruses. Medical Microbiology (8th edition, pp. 503-509). Philadelphia, Pennsylvania: Elsevier Inc. ISBN: 9780323299565
What is Pneumococcal Disease? Pneumococcal disease is a bacterial illness caused by the bacterium Streptococcus pneumoniae. Transmission occurs person-to-person through contact with respiratory droplets or respiratory secretions like mucus. S. pneumoniae is a common inhabitant of the nasal passages with some being asymptomatic carriers. The duration of carriage varies but is generally longer in children than adults. About 20-60% of school-age children and 5-10% of adults are asymptomatic carriers. Infection can range in severity from sinusitis, ear infections, pneumonia, bone and joint infections, bloodstream infections, or meningitis (inflammation of the tissue surrounding the brain and spinal cord). In pneumonia, symptoms usually include an abrupt fever or chills, chest pain, cough that produces mucus, fatigue, increased heart rate, and difficulty breathing. Bloodstream infections can lead to bone and joint infections, meningitis, and endocarditis (inflammation inside the heart) and are fatal in about 10% of cases. Meningitis is characterized by headache, vomiting, fever, stiff neck, hearing loss, seizures, and coma. Pneumococcal meningitis is fatal in about 14% of cases.
What vaccine can help protect me? There are several vaccines available in two different classes of vaccines that protect against pneumococcal disease. UCo Health currently carries Pneumovax 23® (PPSV23), Prevnar 20® (PCV20), PCV15 Vaxneuvance® (PCV15) and Prevnar 13® (PCV13). Prior to major pneumococcal conjugate vaccination in 2000, an estimated 13,000 pneumococcal bloodstream infections and 700 meningitis cases, with 200 child deaths occurred each year in the United States.
How do the vaccines work? PPSV23 contains a set of biomolecules called polysaccharides, which are the building blocks of the protective layer on the outside of the S. pneumoniae bacterium. PPSV23 vaccine protects against 23 types of this bacterium. Vaccination will stimulate the production of antibodies towards the polysaccharide components of the bacterium. PCV13 and PCV20 are categorized as polysaccharide conjugate vaccines which attach the bacterial polysaccharides in the vaccine to a carrier protein to aid in delivery and boost the immune response. PCV13 protects against 13 types of the S. pneumoniae bacterium, while PCV20 protects against 20 types.
Who should get vaccinated? Typically vaccination is recommended in childhood and for all adults 65 years or older.
● Children 2 to 23 months old: Vaccination with PCV13 is recommended and given in 4 doses: Starting at 2 months of age, then 4 months, and 6 months for primary series, then a booster dose at 12 to 15 months.
● Healthy children 24 to 59 months old: Healthy children in this age range should get 1 dose of PCV13 if previously unvaccinated or did not complete their original series. Vaccination is not recommended for those 5 years or older.
● Those 2 years to 64 years old with certain medical conditions: Those in this age range with varying chronic or immunocompromising conditions may require vaccination, but depending on age and condition vaccine brand may vary. Examples of these conditions include: Heart or lung disease, diabetes, liver disease, cerebrospinal fluid leak, cochlear implant, damaged or removed spleen, and autoimmune disorders. If you or your child fall into this category, call UCo Health Disease Prevention Specialist Tanner Pearson at 541-278-6290 to discuss vaccination recommendations based on disease and age.
● Adults 65 years or older: A single dose of PPSV23 is recommended for those in this age range.
Source Information:
CDC Pink Book: Epidemiology and Prevention of Vaccine Preventable Diseases
Textbooks
- Flint, J., Racaniello, V.R., Rall, G.F., Hatziioannou, T., & Skalka, A.M. (2020). Chapter 7 - Vaccines. Principles of Virology: Volume II Pathogenesis and Control (5th edition, pp. 230-258). Washington D.C.: ASM Press. ISBN: 9781683672852
- Murray, P.R., Rosenthal, K.S., & Pfaller, M.A. (2016). Chapter 19 - Streptococcus and Enterococcus. Medical Microbiology (8th edition, pp. 183-201). Philadelphia, Pennsylvania: Elsevier Inc. ISBN: 9780323299565